Continuing Prep for Experiment
- Noreen Mian
- Nov 16, 2017
- 2 min read
The next step was to wash the cells with medium starting at 175microliters to have a constant port volume because for this test, the medium volume does not change. The background wells have the same amount so we can subtract the amount from the background correction to minimize changes. First we have to take out the PBS from the wells and we also don’t put cells in the background because it would interfere. We plated 200μl originally, so we had to remove 100μl from all of the other wells. Then, we added 75μl of medium to dilute the cells. Following, we incubated the cells at 37 degrees. Since the plates had too much medium, we had to dilute them by two in order to get 40μl. We repeated the process of adding 135μl of medium and removing twice and ending with adding in 135μl.
The next step was to use the Seahorse drugs, FCCP, Oligomycin, and Rotenone/ Antimycin A to find the stock volume. This was done by using the port concentration and the total amount we want to have in the solution. For the total solution, we want 500mM.
The calculation we did were:
V1C1=V2C2
For the FCCP, the stock volume was 5.6μl, and we had to add 344μl of medium in order the have a total amount of 350μl.
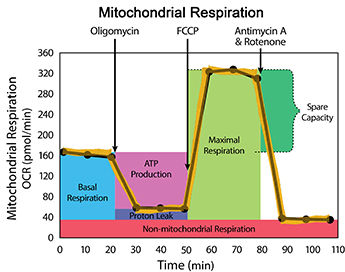
For the Oligomycin, the stock volume was 3.15μl, and we had to add 347μl of medium.
For the Rotenone/Antimycin A, the stock volume was 3.5μl for each, and a total of 7μl, and we had to add 343μl of medium.
We added the drugs into the appropriate tube for the port. First, the medium was added and then the drugs. This was followed by spinning the drugs down to ensure everything was mixed and at the bottom of the tubes.The night before, we hydrated the cartridge plates, so now we added 25 μl of the drugs into each corresponding port on the plate.




Comments